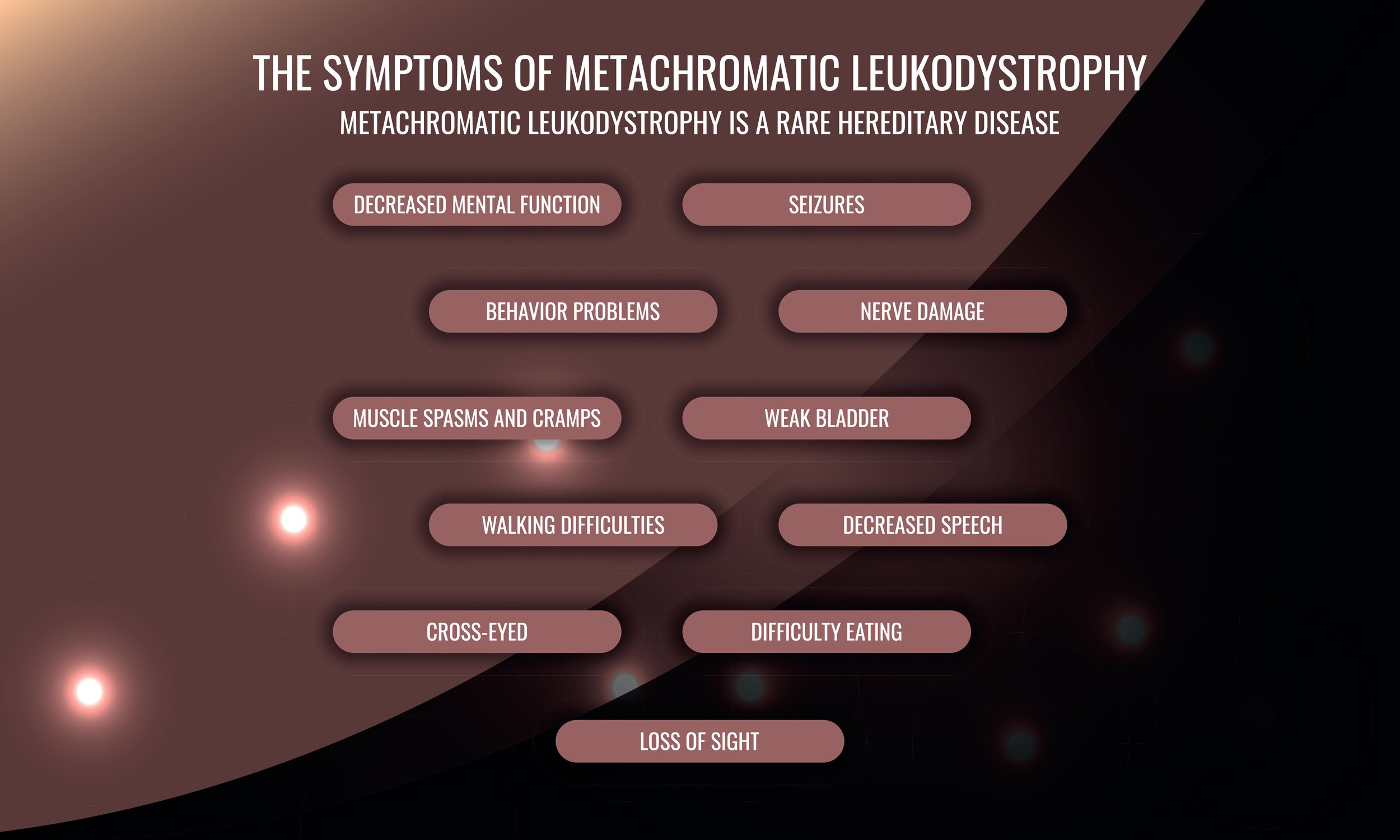
Metachromatic Leukodystrophy (MLD) is a rare recessive genetic disorder caused by a mutation in the arylsulfatase-A (ARSA) gene, which normally breaks down sulfatide, resulting in the toxic buildup of lipids and other storage materials in cells in the white matter of the central nervous system and peripheral nerves. The buildup of storage materials impairs the growth or development of the myelin sheath, the fatty covering that acts as an insulator around nerve fibers.[i] Affected individuals experience progressive deterioration of intellectual functions and motor skills, such as the ability to walk. They develop loss of sensation in the extremities, incontinence, seizures, paralysis, inability to speak, blindness, and hearing loss. Eventually they lose awareness of their surroundings and become unresponsive.[ii]
There are three types of MLD: Late Infantile MLD, Juvenile MLD, and Adult MLD. The most common form is Late Infantile MLD, which typically becomes evident between 6 and 30 months of age. The second type, Juvenile MLD, typically appears between 30 months and 17 years of life. It is broken down further into early juvenile (30 months to 7 years) and late juvenile MLD (ages 7 through 16). Individuals with the third type, Adult MLD, begin to show symptoms between 17 and 40 years of age.[iii],[iv]
Currently, no cure exists for MLD. The treatment is symptomatic and supportive. Bone marrow transplantation may delay progression of the disease in some infantile-onset cases. The prognosis of MLD is poor, most children with the infantile type die by age 5. The other forms of MLD- juvenile progress with death occurring 10-20 years after onset and with the adult form, die within 6-14 years following the onset of symptoms.[v]
The true prevalence rate of MLD is unknown but is estimated to be between 1 in 40,000 and 1 in 160,000. The Navajo also have a higher prevalence rate of 1 in every 2,500 people. In certain populations in the Middle East, these numbers may be even higher.[vi]
Libmeldy (atidarsagene autotemcel; OTL-200) is approved in the European Union (2020), UK, Iceland, Liechtenstein, and Norway as a one-time gene therapy to correct the underlying cause of MLD for eligible patients.[vii] Libmeldy is an ex vivo autologous gene therapy for early-onset MLD. CD34+ cells are transduced ex vivo with a lentiviral vector encoding ARSA cDNA. Orchard Therapeutics has completed its rolling biologics license application (BLA) for priority review of OTL-200 (atidarsagene autotemcel) for treating children with early-onset metachromatic leukodystrophy (MLD). If accepted, the FDA would make a decision on the therapy in the first half of 2024. The FDA has previously granted the therapy Rare Pediatric Disease and Regenerative Medicine Advanced Therapy designations.[viii] Information on the clinical study design, safety and efficacy may be found at https://www.libmeldy.eu/clinical-results/.
In the EU, Libmeldy is indicated for the treatment of metachromatic leukodystrophy (MLD) characterized by biallelic mutations in the arylsulfatase A (ARSA) gene leading to a reduction of the ARSA enzymatic activity:[ix]
In children with late infantile (<30 months) or early juvenile (30 months-7 years) forms, without clinical manifestations of the disease
In children with the early juvenile form, with the ability to walk independently and before the onset of cognitive decline*
*Early symptomatic: treatment with Libmeldy of a patient with an early-symptomatic early juvenile form of the disease should be considered:
If this patient is able to walk independently, which means that the patient’s gross motor function classification (GMFC)-MLD score is ≤1, and
If the patient’s cognitive function has not started declining, which means that the patient’s IQ is ≥85.
On September 20, 2021, the University of Minnesota Masonic Children's Hospital performed the first gene therapy procedure in the United States for a 6-year-old girl with metachromatic leukodystrophy (MLD). The University previously received an approval from the FDA to administer Libmeldy under compassionate use and was the first US clinical trial site to administer the investigational gene therapy.[x]
For more information on compassionate use of Libmeldy, contact medinfo@orchard-tx.com.
Article by Kathy Clark, RN, BSN, CMCN, Vice President, Director of Managed Care. For more information about how this may affect your plan, please contact your Summit ReSources care specialist. The following sources were used as reference material for this article:
[i] Metachromatic Leukodystrophy. Accessed 8/16/2023. https://www.ninds.nih.gov/health-information/disorders/metachromatic-leukodystrophy
[ii] Metachromatic Leukodystrophy. Accessed 8/16/2023. https://rarediseases.info.nih.gov/diseases/3230/metachromatic-leukodystrophy
[iii] Types of MLD – Onset/Progression. Accessed 8/16/2023. https://mldfoundation.org/mld-101-progression.php
[iv] What is MLD. Accessed 8/16/2023. https://med.umn.edu/pediatrics/programs-centers-institutes/leukodystrophy-center/metachromatic-leukodystrophy
[v] Metachromatic Leukodystrophy. Accessed 8/16/2023. https://www.ninds.nih.gov/health-information/disorders/metachromatic-leukodystrophy
[vi] Metachromatic Leukodystrophy. Accessed 8/16/2023. https://rarediseases.org/rare-diseases/metachromatic-leukodystrophy/
[vii] Orchard Therapeutics Announces Publication in The Lancet of Long-term Clinical Outcomes with Libmeldy for the Treatment of Children with Early-onset MLD. Accessed 8/16/2023. https://ir.orchard-tx.com/news-releases/news-release-details/orchard-therapeutics-announces-publication-lancet-long-term
[viii] Johnson,V. BLA Submission Gives Libmeldy a Shot at US Review for Metachromatic Leukodystrophy. Accessed 8/16/2023. https://www.cgtlive.com/view/bla-submission-gives-libmeldy-a-shot-at-us-review-for-metachromatic-leukodystrophy
[ix] Libmeldy Indications. Accessed 8/16/2023. https://www.libmeldy.eu/indication/
[x] Orchard Therapeutics Announces Publication in The Lancet of Long-term Clinical Outcomes with Libmeldy for the Treatment of Children with Early-onset MLD. Accessed 8/16/2023. https://ir.orchard-tx.com/news-releases/news-release-details/orchard-therapeutics-announces-publication-lancet-long-term