Over the last few years, cell and gene therapies have emerged as new ways to treat and sometimes cure diseases which previously had no treatment. Although rare diseases and oncology dominate the cell and gene therapy pipeline, the results of the thousands of ongoing clinical trials also identify common diseases linked to gene disorders that may be potential targets for these therapies. Such targets include Alzheimer’s, HIV, cardiovascular disease, and arthritis.(1)
Why Cell and Gene Therapy?
Genetic diseases are caused by spontaneous or inherited mutations in genes which result in one or more proteins with limited or no function. The diagram below provides an overview of several disorders and their related chromosomes.
The goal of cell and gene therapy is to restore gene function to allow for proteins to be properly produced. While therapy can often “cure” a person with the disease, it does not correct the mutated DNA of the reproductive cells. Thus, the person can still pass the genetic disease on to his or her children.
Cell Therapy
Cell therapy refers to the transplantation of human cells to replace or repair damaged tissue and/or cells. Advances in research continue to identify many different types of cells that may be used as part of a therapy or treatment for a variety of diseases and conditions. Some of the cells that may be used include hematopoietic (blood-forming) stem cells (HSC), skeletal muscle stem cells, mesenchymal stem cells, lymphocytes, dendritic cells, and pancreatic islet cells. Hematopoietic stem cell transplantation (also called bone marrow transplant) is the most frequently applied cell therapy and is used to treat a variety of blood cancers and hematologic conditions. Potential applications of cell therapies include treating cancers, autoimmune disease, urinary problems, and infectious disease, rebuilding damaged cartilage in joints, repairing spinal cord injuries, improving a weakened immune system, and helping patients with neurological disorders.(2)
One of the newest approved cell therapies is Chimeric Antigen Receptor (CAR) T-Cell therapy. An individual’s T-cells are removed, sent to a lab, and altered to add a receptor to the T-cells that attracts and kills cancer cells.(3) See Figure 2 for details.
Gene Therapy
Gene therapy aims to add, delete, or correct genetic material in order to treat a disease. The addition, deletion, or correction of genetic material changes how a cell produces a protein or group of proteins. This change gives the cell a new set of instruction that can help treat the disease at the genetic level.
There are two main types of gene therapy being studied: gene addition and gene editing. As its name implies, gene addition adds genetic material to a person’s cells. Alternately, gene editing includes gene insertion, gene inactivation (also called silencing), and gene correction. Gene correction is a technique used to recognize and form a break in the DNA, insert new genetic material, and override the faulty gene, as illustrated below.(4)
Historical Perspective and Current Status
Though FDA approval of cell and gene therapies is relatively recent, there is a long history leading up to the treatment modalities of today. Following is a timeline of progress in gene-based therapies.(5)
Cell and gene therapy FDA approval in the U.S. started out more slowly than other countries but has taken off in the last few years. Pharmaceutical Research and Manufacturers of America (PhRMA) recently reported nearly 400 therapies in Phase I-III clinical trials, with 50% of these aimed at cancer and roughly 30% for rare diseases.(6) In response to the increase in applications for cell and gene therapy reviews, the FDA is offering accelerated approval and regenerative medicine advanced therapy (RMAT) designations and has created a specific set of guidelines for manufacturers. The FDA anticipates approving as many as 20 cell and gene therapies annually by 2025.(7)
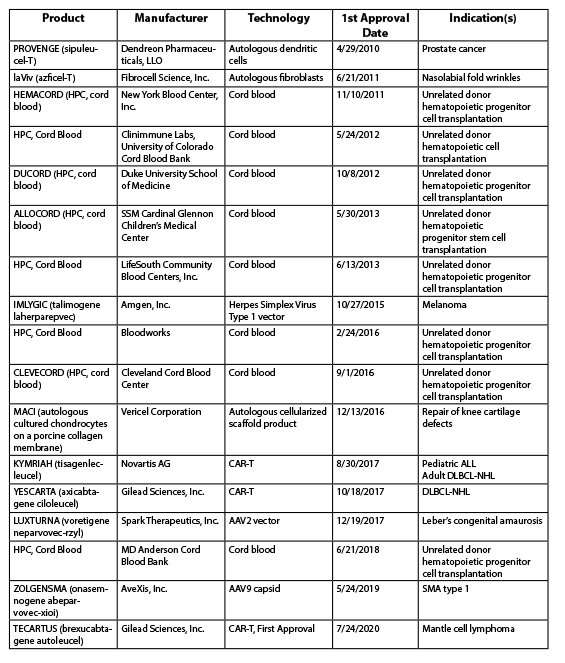
As of November 2020, the following cell and gene therapies had been approved in the US:
Challenges
As noted earlier, cell and gene therapies offer treatment—and sometimes a cure—for individuals with rare or unique conditions that historically had neither. Many of these therapies treat rare diseases which affect a limited number of individuals. In addition, the development of such therapies can take years and consume millions of research dollars. It is therefore not surprising that the price tag for cell and gene therapies is often extremely high for manufacturers to realize a return on their investment. For payers, the extremely high price tags raise red flags and the huge, one-time cost presents financial challenges. On the other hand, manufacturers argue that the one-time, upfront cost is worth it as individuals are now “cured,” and thus no longer need ongoing therapy. That argument would be stronger if individuals stayed with a single insurance company throughout their life. However, in the U.S., individuals change insurance companies with relative frequency, so the long-term savings would not be realized by a single payer.(8)
Another significant concern is payment of an extremely high-cost therapy based on clinical trials of a small population and unknown long-term efficacy. It raises questions from payers regarding the true length of therapeutic benefit and whether additional treatment will still be necessary. One-time, extremely high cost therapy has evoked considerable discussion from manufacturers, pharmacy benefit managers (PBM) and payers regarding payment options. The table below outlines some of the proposed payment models being discussed.(9)
Valuation of Gene and Cell Therapies
Defining the value of these new cell and gene therapies presents a significant challenge, and it is further intensified due to the extremely high price tags. Several factors complicate the analysis. For example, as more therapies come to market, the role of societal pressure in determining their value continues to increase. In addition, payers must consider the cost of a one-time therapy compared to the cumulative lifetime cost of currently available therapies. Furthermore, new metrics may be applied when considering whether an individual is an appropriate candidate for a therapy; these may include severity of disease, age of disease onset, lifetime burden of illness, benefits of return to work, and increased societal productivity.(10) Finally, several cell and gene therapies will be targeting younger individuals with severe conditions. Will treatment of children be given greater value than treatment of adults? Who defines how much “better” an individual must be to warrant giving them the cell or gene therapy? Regardless of how the answers shake out, the one thing that remains certain is that these therapies will continue to be extremely expensive to develop and manufacture for the foreseeable future.
Navigating the Challenging Landscape
Now that cell and gene are coming to market on a regular basis, even a single treatment can become a significant pain point for payers and employer groups across the country. In response to this challenge, Summit Re has dedicated resources to understanding the cell and gene therapy and specialty pharmacy landscapes. As a result, the company developed the ReAssureRx program to help clients address the potentially devastating expenses. ReAssureRx is comprised of a variety of pharmacy cost containment options that can be customized to fit a payer’s pharmacy review processes and contracts. The program is designed to protect the payer’s bottom line against catastrophic pharmacy claims through partnerships formed with industry leaders in cell and gene therapy as well as specialty pharmacy.
Summary
Cell and gene therapy will most certainly continue to shift paradigms for manufacturers, patients, healthcare providers and pharmacies for many years. These are groundbreaking technologies which offer hope to millions of individuals that previously had no viable options for treatment or cure. By working together with Summit Re, payers and employer groups can tackle head on the challenges of the cell and gene therapy market.
About the author: Debbie Hoffer, RN, MS, CCM, is vice president and managed care consultant of Summit Reinsurance Services, Inc. She can be reached at dhoffer@summit-re.com.
Citations
(1) Institute for Clinical and Economic Review. GENE THERAPY: Understanding the Science, Assessing the Evidence, and Paying for Value. Accessed March 19, 2020 from https://icer-review.org/wp-content/uploads/2017/03/ICER-Gene-Therapy-White-Paper-030317.pdf.
(2) https://www.aabb.org/news-resources/resources/cellular-therapies/facts-about-cellular-therapies. Accessed January 20, 2021.
(3) https://www.aabb.org/news-resources/resources/cellular-therapies/facts-about-cellular-therapies. Accessed January 20, 2021.
(4) https://www.thegenehome.com/how-does-gene-therapy-work. Accessed January 15, 2021.
(5) A Historical Look at Gene-Based Therapies. Accessed January 7, 2021 from https://www.exploregenetherapy.com/history-of-gene-replacement-therapy.
(6) America’s Biopharmaceutical Companies Medicines in Development 2020 Report. Published on March 10, 2020.
(7) FDA prepares for expected surge in gene therapy trials. Pharmaceutical Technology. Published February 5, 2019. Accessed December 12, 2020. Available at https://www.pharmaceutical-technology.com/comment/fds-gene-therapy.
(8) Institute for Clinical and Economic Review. GENE THERAPY: Understanding the Science, Assessing the Evidence, and Paying for Value. Accessed March 19, 2020 from https://icer-review.org/wp-content/uploads/2017/03/ICER-Gene-Therapy-White-Paper-030317.pdf.
(9) Datamonitor Healthcare payer research; In vivo 2017, 2018; Institute for Clinical and Economic Review, 2017; Managed Healthcare Executive, 2018; Penn Medicine News, 2014, Reuters, 2015.
(10) Adrienne Brennan, PharmD Marcie Morris, PharmD. Gene and Cell Therapy: A New Age of Medicine. AllianceRx 2020.